Challenges to diagnosing EPI
Diagnosing EPI can be difficult due to several factors2:

The signs and symptoms of EPI often overlap with those of other GI conditions.3

EPI can be present in a number of underlying conditions and procedures.3

EPI can be transient depending on underlying etiology.4,5

EPI may not be present until months or years after the underlying condition or procedure.6-8

Current diagnostic testing for EPI is limited and inconvenient.9

An AGA online survey showed patients waited an average of 4 years to see a doctor about their GI symptoms.10*
- 60% of them held off due to embarrassment of discussing symptoms
1 in 4 patients had their diagnosis changed to EPI after initially being diagnosed with another GI condition.10*
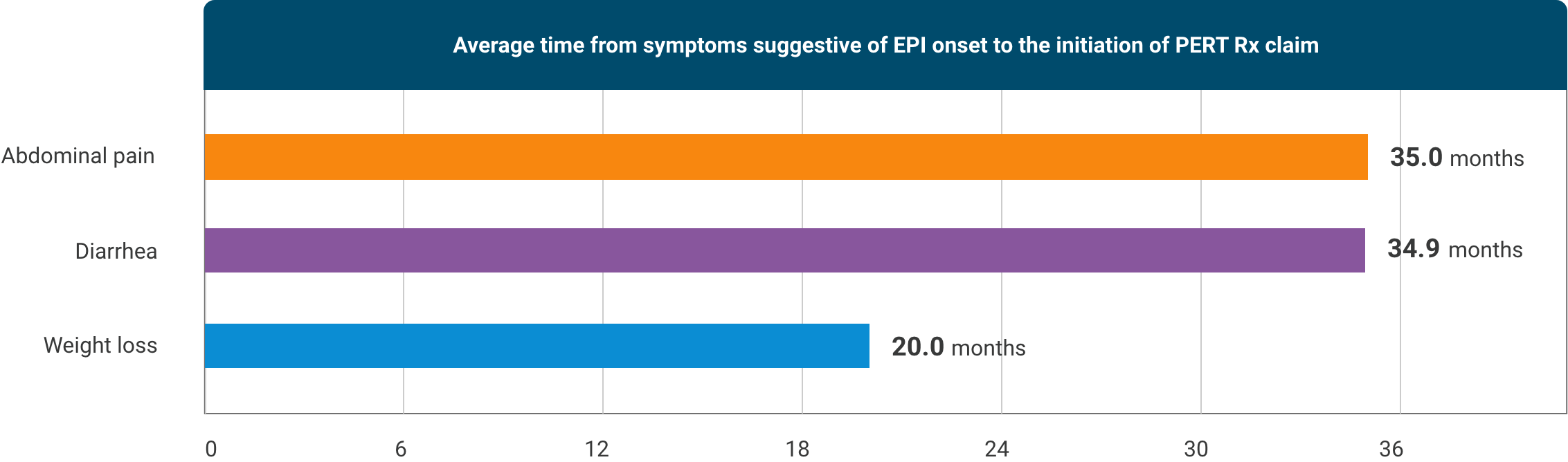
Some symptoms suggestive of EPI may be present up to 3 years before PERT initiation11†
Consider PERTs—the standard of care for EPI treatment—as part of your comprehensive EPI management plan.2,12
*EPI Uncovered is based on an online survey conducted by Harris Poll from May 17 through June 20, 2016. It included 1,001 US adults ages 18 and older who experienced at least 2 gastrointestinal issues 3 or more times in the past 3 months (“patients”), as well as 250 primary care physicians (PCPs) and 250 gastroenterologists in the United States who are ages 18 years or older and licensed. Figures for patients were weighted where necessary based on age, education, gender, race/ethnicity, region, income, size of household, marital status, and likelihood to be online to bring them into line with their actual proportions in the population. Figures for PCPs and gastroenterologists were weighted on years in practice, gender, and region, where necessary, to bring them into line with their actual proportions in the population.
†Retrospective study to quantify the time between the presence of symptoms suggestive of EPI prior to initiating a PERT. To be eligible for the study, a patient must: 1) have a history of at least 1 medical claim in each year during the 5-year study period (between 2014 and 2019), 2) have at least 1 EPI diagnosis during this period, and 3) have initiated PERT in 2019. Time to PERT was based on the first occurrence of the symptom code prior to PERT prescription claim.
AGA = American Gastroenterological Association; EPI = exocrine pancreatic insufficiency; GI = gastrointestinal; PERT = pancreatic enzyme replacement therapy.
