CREON clinical efficacy
In clinical trials, CREON demonstrated efficacy in patients with exocrine pancreatic insufficiency (EPI) due to chronic pancreatitis, pancreatectomy, and cystic fibrosis.1
Chronic Pancreatitis and Pancreatectomy Pivotal Trial1,2
CREON was studied in patients aged 32 to 75 years old with EPI due to chronic pancreatitis (CP) and pancreatectomy.
CREON achieved statistically significant improvement in CFA from baseline at day 7 of treatment vs placebo (P<0.0001)1,2
Primary endpoint: Change in CFA from baseline to the end of the double-blind treatment period
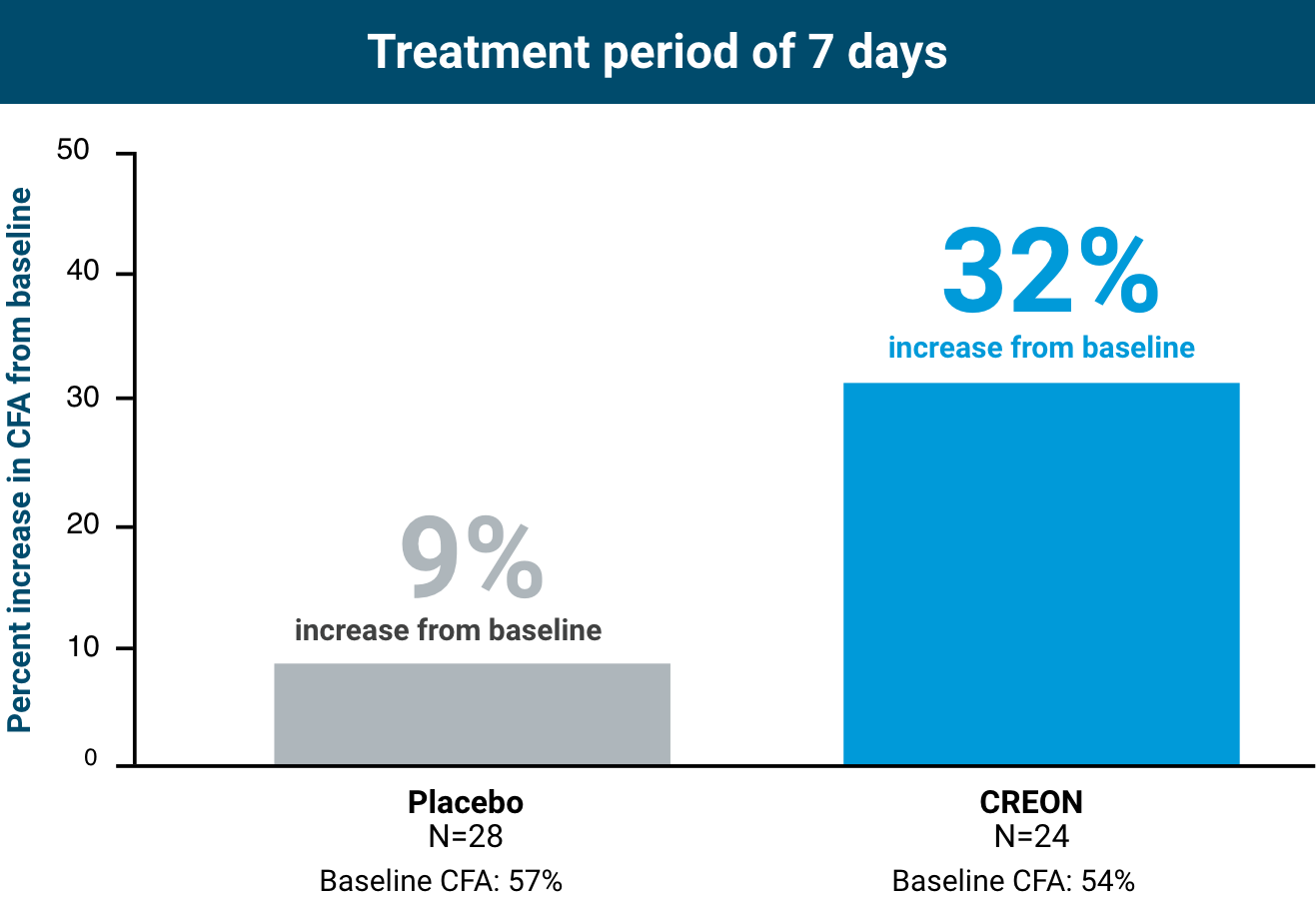
CFA at day 7: Placebo: 66%; CREON:
- Mean treatment difference in CFA was 21% (P<0.0001)
- In healthy subjects, CFA is approximately ≥93%3
Safety Considerations
- Adverse reactions that occurred in at least 1 chronic pancreatitis or pancreatectomy patient (greater than or equal to 4%) receiving CREON were hyperglycemia, hypoglycemia, abdominal pain, abnormal feces, flatulence, frequent bowel movements, and nasopharyngitis
Cystic Fibrosis Pivotal Trial (Subjects Aged 12 to 43 Years)1,4
CREON was studied in patients aged 12 to 43 years with EPI due to cystic fibrosis (CF).
Patients taking CREON achieved statistically significant improvement in fat absorption after 5-6 days of treatment
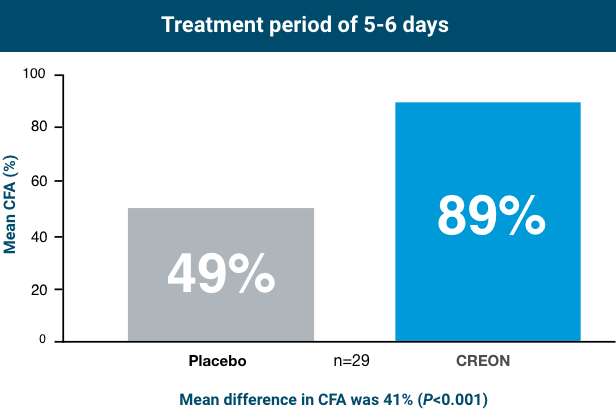
In healthy subjects, CFA is approximately ≥93%.3
Safety Considerations
- Adverse reactions that occurred in at least 2 cystic fibrosis patients (greater than or equal to 4%) receiving CREON were vomiting, dizziness, and cough
Patients taking CREON achieved statistically significant improvement in protein absorption after 5-6 days of treatment
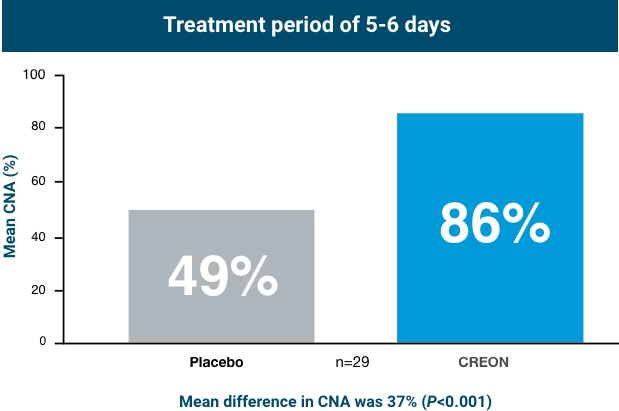
In healthy subjects, CNA is approximately 88%.3
Safety Considerations
- Adverse reactions that occurred in at least 2 cystic fibrosis patients (greater than or equal to 4%) receiving CREON were vomiting, dizziness, and cough
Cystic Fibrosis Pivotal Trial (Subjects Aged 7 to 11 Years)1,5
CREON was studied in pediatric patients aged 7 to 11 years with EPI due to cystic fibrosis (CF).
Patients taking CREON achieved statistically significant improvement in fat absorption after 5-6 days of treatment
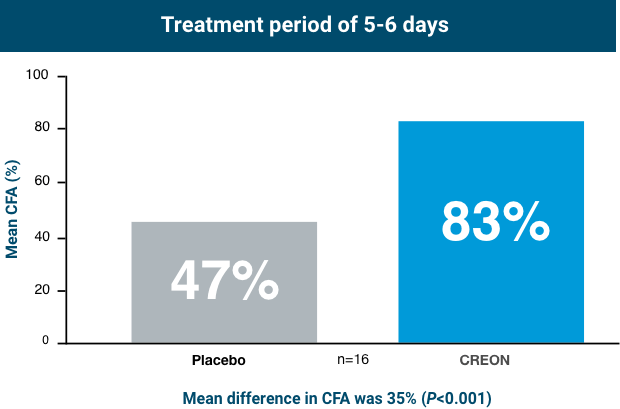
In healthy subjects, CFA is approximately ≥93%.3
Safety Considerations
- Adverse reactions that occurred in at least 2 cystic fibrosis patients (greater than or equal to 4%) receiving CREON were vomiting, dizziness, and cough
Patients taking CREON achieved statistically significant improvement in protein absorption after 5-6 days of treatment
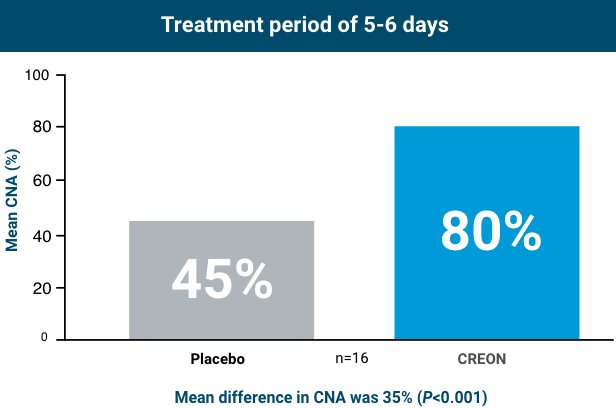
In healthy subjects, CNA is approximately 88%.3
Safety Considerations
- Adverse reactions that occurred in at least 2 cystic fibrosis patients (greater than or equal to 4%) receiving CREON were vomiting, dizziness, and cough
Based on the clinical studies included in the FDA-approved label, CREON has a well-characterized safety profile.1
CFA = coefficient of fat absorption; CNA = coefficient of nitrogen absorption; FDA = Food and Drug Administration.
